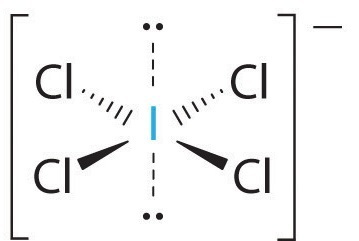
First, you have to see the Lewis structure of the compound as shown in the attached picture. As we can observe, there are lone pairs of electrons surrounding I. So, the first choice is not the answer. Its molecular geometry is square planar, which means the bond angle is 90°. So, the second choice is not the answer. The electron domain of ICl₄ is octahedral. So, the third choice is correct. The fourth choice is wrong. Therefore, the answer is C.